TumorSelect® mechanism of action has potential indications for enhanced safety and efficacy of therapy by selective delivery to tumor tissues include the following cancers:
- Breast
- Colorectal
- Gastric
- Glioblastoma
- Leukemia
- Melanoma
- Non-small cell lung
- Ovarian
- Pancreatic
- Prostate
Biological Data
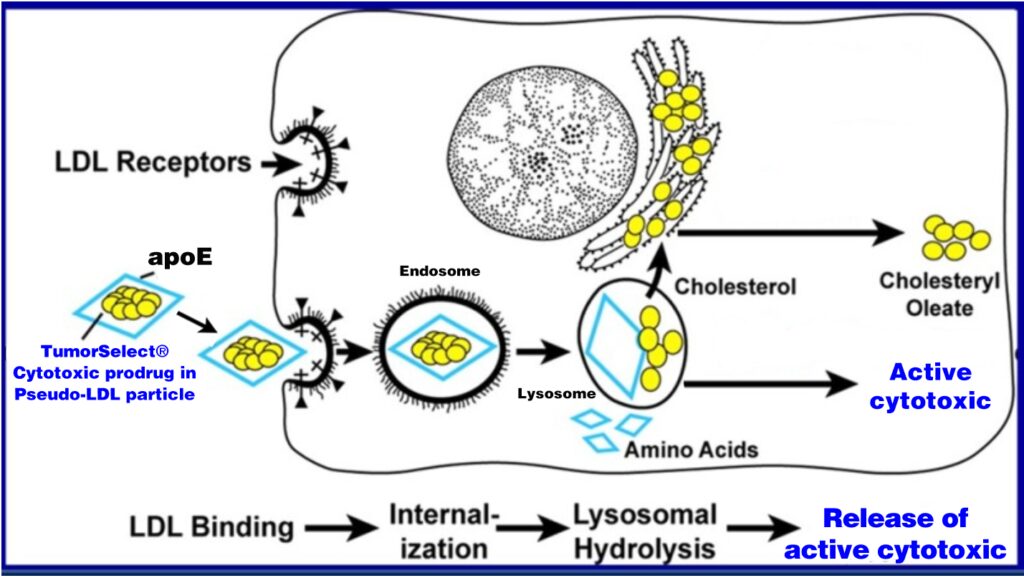
TumorSelect® Mechanism of Action
Tumors are voracious consumers of cholesterol both as an energy source as well as a substrate for rapid cell membrane production. Incorporating chemotherapeutics into pseudo LDL nanoparticles for delivery via overexpressed LDL receptors provides the opportunity for selective delivery of lipophilic chemotherapeutics to tumors compared with normal tissues. Pseudo LDL particles can increase the utility of clinically approved chemotherapeutic agents by altering their PK/PD properties and dramatically raising the therapeutic indices of chemotherapies.
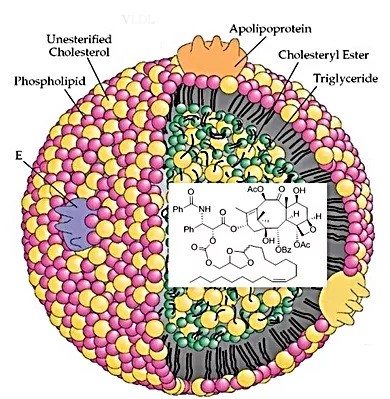
When injected, TumorSelect® pseudo LDL particles accumulate in tumor tissues by the EPR effect where they can be recognized by tumor cell LDL receptors. The particles are internalized into endosomes where the acidic environment causes the breakdown of the particle and the release of free drug into the cytoplasm.
Sequential steps in the LDL receptor pathway of mammalian cancer cells showing uptake of TumorSelect®:
TumorSelect® particles increase the utility of clinically approved chemotherapeutic agents by:
- Increasing plasma residence time
- Targeting chemotherapeutic to cancer cells
- Minimizing distribution into healthy tissues
- Raising therapeutic indices
Proof of Concept for TumorSelect® Delivery of Paclitaxel
Dose Response Study in Murine Model of Human Breast Cancer
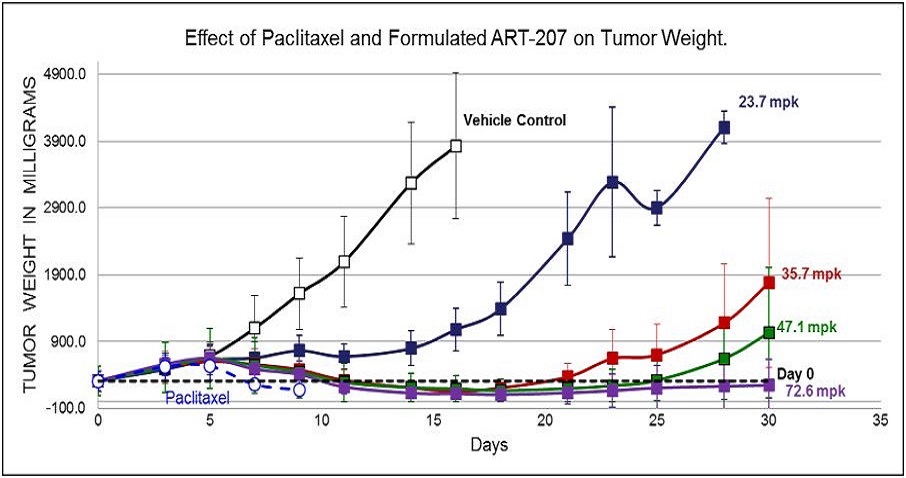
Increasing dose shows increasing efficacy and duration of tumor suppression.
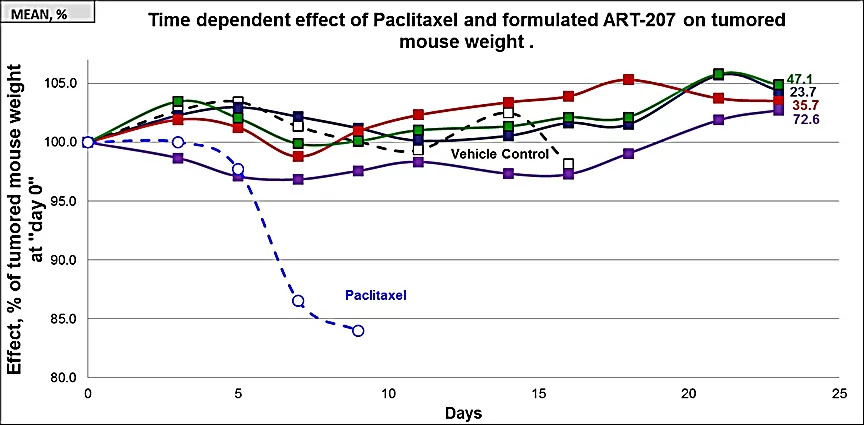
Mice were divided into 6 groups (5 mice in each group). All test articles were administered to mice for the five consecutive days via intravenous (iv) injections. Group #1 received drug-free lipid formulation ([0], open squares) and groups 2-5 received 23.7, 35.7, 47.1, and 72.6 mg/kg of formulated ART-207 (filled squares). Group #6 received 15 mg/kg of Paclitaxel (open circles). Effect is expressed as % of mean mouse weight assessed for each group prior to the first treatment at Day 0. Four of 5 mice died of toxicity in the paclitaxel group before day 10.
Even at highest dose administered of TumorSelect® Paclitaxel (72.6 mg/kg of 207 = 3.4 x MTD of Taxol®) no toxicity significantly different from vehicle control (no cytotoxic content) is observed.
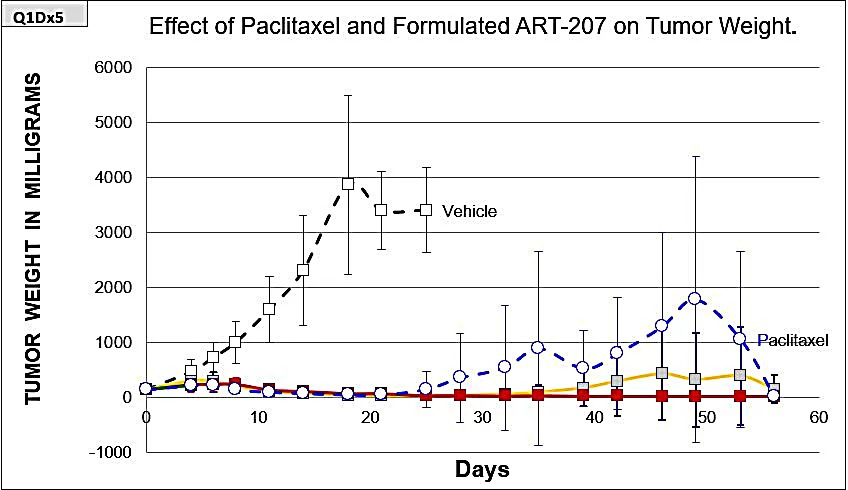
Mice were divided into 5 groups (10 mice in each group). All test articles were administered to mice for five consecutive days via intravenous (iv) injections starting from day 0. Groups #2-4 received 105.2 (solid green), 78.9 (solid red), and 52.6 (solid yellow) mg/kg of formulated ART-207. Group #5 received drug-free lipid formulation (black dotted line, open squares). Group #6 received 15 mg/kg of Cremophor EL/EtOH paclitaxel (blue dotted line, open circles).
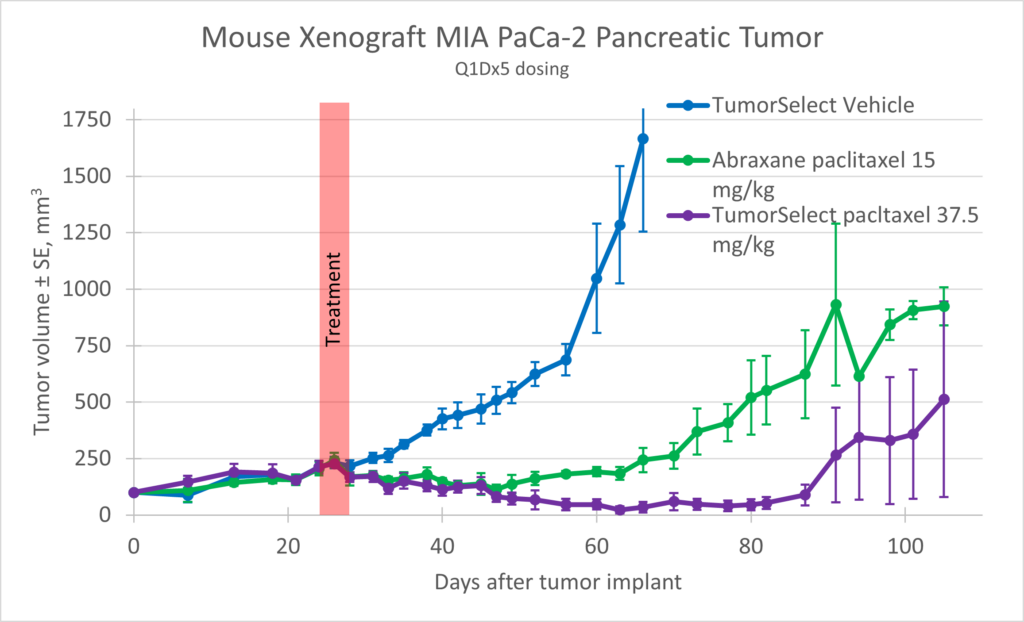
MIA-PaCa-2 (ATCC, Manassas, VA) was implanted in NOD scid (NSG) female mice, and the animals were dosed with TumorSelect® paclitaxel with a comparison positive control of Abraxane®
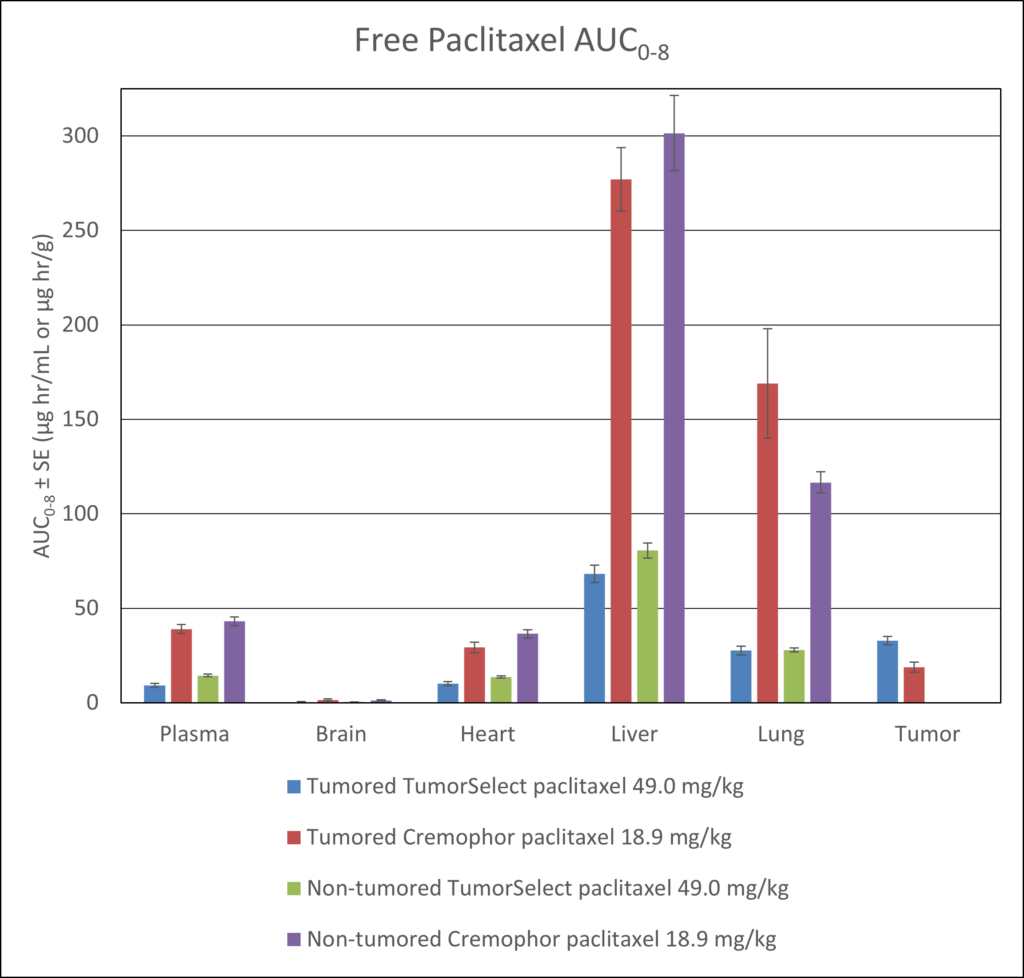
TumorSelect® Paclitaxel vs. Taxol® and Abraxane®
Overall TumorSelect® paclitaxel toxicity, efficacy and PK data show:
- Tumor growth suppression
- Less toxicity allows higher dose intensity
- Slower tumor regrowth / increased duration of efficacy
- Increased survival
- Higher number of tumor-free animals
- Significantly lower concentrations of paclitaxel in non-target tissues
- Significantly higher concentrations of paclitaxel in tumor tissue
- The time-averaged delivery (intact prodrug plus free paclitaxel) is 3.27% of the prodrug dose at 480 minutes